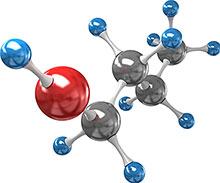
Computational theory revealed the reaction rates for hydrogen atom (blue) abstraction during combustion at each of the five hydrogen binding sites on 1-butanol. The star players in butanol combustion are the hydrogens bonded to the carbon (grey) immediately next to the oxygen (red), which let go of butanol far more readily than the other hydrogens.
New atomic-level details about how butanol burns are making combustion chemistry models more accurate and helping design fuel systems that burn more efficiently and cleanly. Butanol (C4H9OH), is an advantageous alternative to ethanol fuel that can be derived from plants. It stores a great deal of chemical energy in its hydrogen bonds, which are located at five sites—four where hydrogen atoms attach directly to the butanol carbon backbone and one where hydrogen attaches as part of a hydroxyl, or OH, group. With the help of supercomputing resources, including EMSL’s Chinook, researchers studied how these bonds break—releasing a burst of energy—during combustion such as in a vehicle engine. Specifically, they focused on a critical step in the high-temperature combustion process when hydrogen atoms in 1-butanol break away, joining hydroxyl radicals (OH•) to form water. The research team used multistructural variational transition state theory, or MS-VTST, to calculate the reaction rates of this hydrogen abstraction—in other words how readily hydrogen abstraction proceeds—over a range of temperatures for each of the five hydrogen bonding sites. The sum of the five computed reaction rates, or the overall rate, was compared to the overall rate measured experimentally and found to be in excellent agreement. This agreement lends confidence that the five computed reaction rates, which cannot be measured individually using experimental means, are accurate. Adding this fine level of detail about reaction rates to combustion models helps guide chemists and engineers toward fuel systems that maximize energy output and minimize combustion byproducts such as carbon monoxide and soot.
Reference: Seal P, G Oyedepo, and DG Truhlar. 2012. “Kinetics of the Hydrogen Atom Abstraction Reactions from 1-Butanol by Hydroxyl Radical: Theory Matches Experiment and More.” Journal of Physical Chemistry A 117:275−282. DOI: 10.1021/jp310910f.
Participants: University of Minnesota
Acknowledgments: This work was supported in part by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences as part of the Combustion Energy Frontier Research Center. Some of the computations were performed at EMSL, a national scientific user facility sponsored by the DOE Biological and Environmental Research program.
