• connect to article at C&E News (requires institutional or personal subscription)
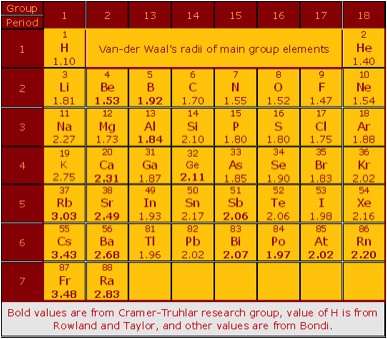
| Consistent van der Waals Radii | |
 |
Atomic radii are not precisely
defined but are nevertheless widely used
parameters in modeling and understanding molecular structure and
interactions. The van der Waals radii determined by Bondi from
molecular crystals and data for gases are the most widely used values,
but Bondi recommended radius values for only 28 of the 44 main-group
elements in the periodic table. In work carried out by postdoctoral
research associates Manjeera Mantina and Rosendo Valero, graduate
student Adam Chamberlin, and Professors Christopher Cramer and Donald
Truhlar, atomic radii for the other 16 were presented; these new radii
were determined from electronic structure calculations on selected van
der Waals molecules in a way designed to be compatible with
Bondi’s scale. The method chosen is a set of two-parameter
correlations of Bondi’s radii with repulsive-wall distances
calculated by relativistic coupled-cluster electronic structure
calculations. The newly determined radii (in Å) are Be, 1.53; B,
1.92; Al, 1.84; Ca, 2.31; Ge, 2.11; Rb, 3.03; Sr, 2.49; Sb, 2.06; Cs,
3.43; Ba, 2.68; Bi, 2.07; Po, 1.97; At, 2.02; Rn, 2.20; Fr, 3.48; and
Ra, 2.83. Bondi also determined van der Waals radii for several
transition metals and one actinide; if we combine his radii for these
elements with the 44 main-group radii, we have a consistent set of
radii for 54 elements. |
| A science & technology concentrated news item describing this work was written by Elizabeth Wilson and appears in the May 11, 2009 issue of Chemical & Engineering News. | |
| • download this
article as a .webarchive file • connect to article at C&E News (requires institutional or personal subscription) |
|