

 |
 |
Comp Chem Research Developments | |
| Archive of Comp Chem Research News | |
| May 11th, 2005 | |
| Tuning the Diradical Character of meta-Arynes | |
|
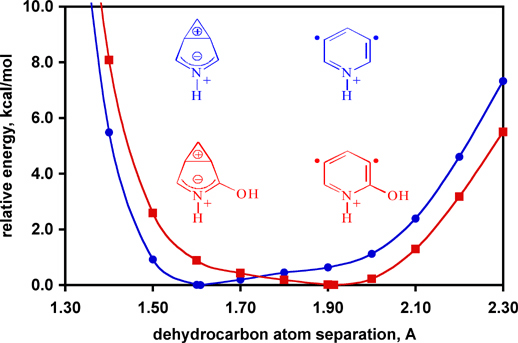
Aryne diradicals are the reactive intermediates responsible for DNA cleavage in the enediyne class of antitumor-antibiotics (e.g., esperamicin, shown at right complexed with double-helical DNA). Naturally occurring diradicals tend to belong to the para-aryne family as other regioisomers are less reactive. A combined computational (Professor Christopher Cramer of the University of Minnesota) and experimental (Professor Hilkka Kenttämaa of Purdue University) study on the gas-phase structures and reactivities of charged meta-arynes has demonstrated for the first time that the reactivity of these congeneric biradicals can be "tuned" to be even greater than that of para-arynes by using appropriate substituents. These substituent effects are not the result of changes in the singlet-triplet gaps of the biradicals, but rather in the potential energy surfaces for the dehydrocarbon separation as illustrated below. Further details are available in a Communication to the Editor of the Journal of the American Chemical Society (Nash, J. J.; Nizzi, K. E.; Adeuya, A.; Yurkovich, M. J.; Cramer, C. J.; Kenttämaa; H. I. "Demonstration of Tunable Reactivity for meta-Benzynes" J. Am. Chem. Soc. 2005, 127, 5760 [pdf]) |
|
|
|
|
|
|
| |