

 |
 |
Comp Chem Research Developments | |
| Archive of Comp Chem Research News | |
| November 10th, 2004 | |
| Dioxygen Activation at a Single Copper Site | |
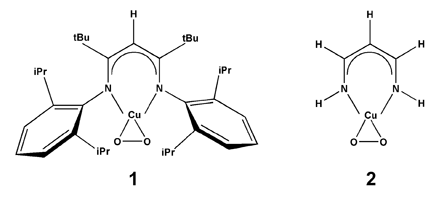
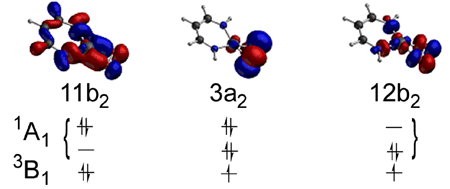
The formation of 1:1 Cu/O2 adducts is an early step in the catalytic cycles of neurologically important binuclear uncoupled copper-containing enzymes, such as dopamine β-monooxygenase (DβM) and peptidylglycine α-hydroxylating monooxygenase (PHM). Earlier computations1 surveying a variety of η2 1:1 metal/O2 complexes concluded that the degree of charge transfer from the metal to the O2 moiety can be variable, resulting in a continuum of species ranging from superoxide to peroxide. A subsequent paper2 contended on the basis of density functional theory (DFT) calculations, however, that, for the case of an η2 Cu/O2 complex supported by a simplified β-diketiminate ligand (Figure 1), such a continuum does not exist and rather that the two states of CuIII-peroxide and CuII-superoxide are distinct, differing in symmetry and multiplicity. In a recently published article in Inorganic Chemistry, 2004, 43, 7281, post-doctoral fellow Benjamin Gherman and Professor Christopher J. Cramer employed a combination of DFT and multireference second-order perturbation theory (CASPT2) methods in order to settle the debate regarding the nature of O2 binding in 1:1 Cu/O2 complexes, specifically in those with the β-diketiminate ligand. While DFT was found to yield satisfactory geometries and charge populations in all cases, multideterminantal character in the biradical-like CuII-superoxide mesomer (Figure 2) caused DFT to fail badly in predicting these species' energies, necessitating the use of CASPT2 calculations. Using a computational protocol combining the strengths of DFT and CASPT2, a smooth continuum was found between CuII-superoxide and CuIII-peroxide character with variation of the electron donor/acceptor characteristics of the supporting β-diketiminate ligand. The results reinforced the validity of the earlier conclusions from Cramer and co-workers1 and illustrated the limitations of DFT in modeling biradical-like systems. | |
| |
|
Figure 1. The 1:1 η2 Cu/O2 complexes supported by the full (1) and simplified (2) β-diketiminate ligand. | |
| |
|
Figure 2. Key frontier orbitals showing partial occupation in the singlet and triplet CASSCF wave functions. The multideterminantal character of the singlet can lead to significant errors in the DFT energies as the contributions of the two configurations to the singlet wave function become more equal (e.g. near the CuII-superoxide side of the dioxygen-binding continuum). | |
| * This page is updated every two weeks. Next scheduled update: Nov 24th, 2004. | |
|
| |