The ability to tune lasers to specific energies suggests the possibility of microscopic control of chemical dynamics. Molecules in excited electronic states are highly energetic and are not significantly populated in most thermally equilibrated systems. Electronically excited species can be prepared using lasers, and these excited species are more reactive than ground-state species, using their electronic energy to form and break chemical bonds. By carefully tuning a series of laser pulses and controlling the distribution of electronic energy among the various bonds, it may be possible to prepare electronically excited reactants that preferentially form specific products in ratios different from those obtained using thermal energy to prepare the reactants. Understanding how a molecular system absorbs and disposes of photon energy is the first step toward understand and developing laser-controlled chemical reactions.
The Li...FH system is an ideal candidate for the theoretical study of laser-controlled chemistry; it is simple enough to treat using ab initio quantum mechanics, yet it is complex enough to have competing dissociation dynamics. We have developed accurate global representations of the ground and first-excited electronic states of LiFH. The photoabsorption spectrum of the Li...FH van der Waals complex and the resulting photodissociation dynamics have also been studied.
Visit the Truhlar Group web page
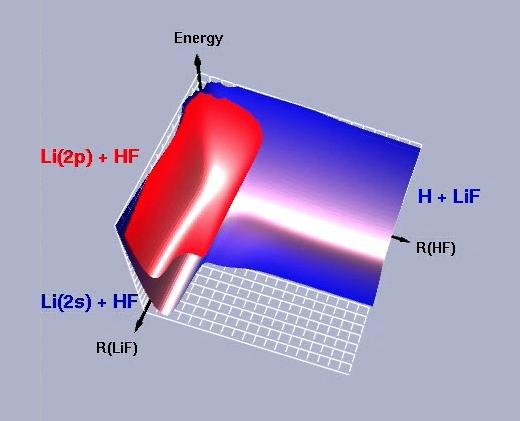
Trajectory surface hopping simulations:
| Click here to view a reactive event forming H + LiF | Click here to view a nonreactive event forming Li + HF |