

 |
 |
Comp Chem Research Developments | |
| Archive of Comp Chem Research News | |
| May 28, 2003 | |
|
| |
|
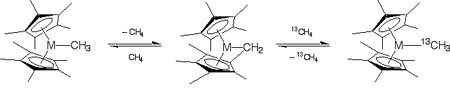
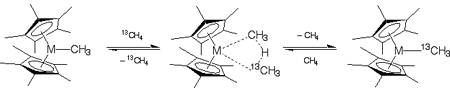
Functionalization of unsubstituted hydrocarbons is a long-standing goal of organometallic chemistry. bis(Pentamethylcyclopentadienyl)methyllutetiocene has been demonstrated experimentally to catalyze CÜH bond metathesis in methane (a particularly cheap unsubstituted hydrocarbon). In a recently published article in Organometallics, 2003, 22, 1682, graduate student Edward C. Sherer and Professor Christopher J. Cramer employed density functional theory to characterize the mechanistic details of the reaction for supported Lu, Sc, and Y. In particular, they established that accurate modeling of the kinetics of methane metathesis required (i) complete representation of the pentamethylcyclopentadienyl rings and (ii) accounting for quantum mechanical tunneling in the kinetics associated with the activated complex. While a bimolecular metathesis reaction was strongly favored for Y and Lu, a two-step process involving a tuck-in complex intermediate was predicted to be energetically accessible for M = Sc. | |
   | |
|
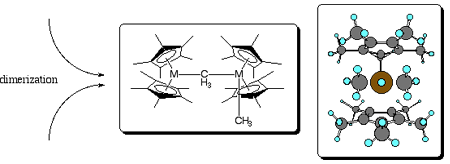
Figure. Methane metathesis pathways involving a tuck-in process (above) or a bimolecular exchange (below), where metathesis is confirmed experimentally by use of 13C-labeled methane. The reactive metallocene is also subject to dimerization as shown at center. The computed transition state structure for the bimolecular process is shown at center right, with H atoms blue, C atoms gray, and Lu brown. | |
|
|