

 |
 |
Comp Chem Research Developments | |
| Archive of Comp Chem Research News | |
|
October 2, 2002 |
|
|
|
In order to understand how dioxygen is activated at copper sites in biological and industrial catalytic systems, the reactions of discrete Cu(I) complexes with O2 have been studied extensively. Typically, an initial 1:1 Cu/O2 adduct either is presumed or established by transient spectroscopy, but efforts aimed at the isolation and characterization of such adducts have been hindered by their tendency to react with a second Cu(I) ion to yield dinuclear species. In an article that recently appeared in J. Am. Chem. Soc. (2002, 124, 10660), graduate students Nermeen W. Aboelella, Anne M. Reynolds, and William W. Brennessel, postdoctoral associate Elizabeth A. Lewis, and Professors Christopher J. Cramer and William B. Tolman report the X-ray crystallographic and density functional theoretical characterization of a novel 1:1 Cu/O2 complex (1) that features side-on (h2) coordination of the O2 unit (Figure, a and b). The data suggest significant contribution of a Cu(III)-(O22-) resonance form, an unusual bonding description for such Cu/O2 species. Because O2 binding in the complex is irreversible at low temperature, addition of Cu(I) reagents to the adduct results in the formation of bis(m-oxo)dicopper(III) complexes that are uniquely asymmetric as a result of the presence of different N-donor ligands on each metal ion (Figure, c). This demonstration of the use of an isolable 1:1 Cu/O2 adduct as a synthon for building multicopper species in stepwise fashion provides important precedent for future applications of the methodology to other systems, including those containing alternative metal ions. |
|
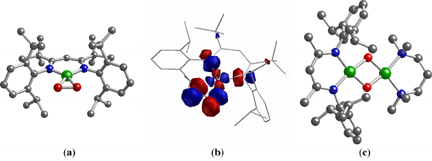
|
|
Figure. (a) X-ray crystal structure of 1:1 Cu/O2 complex 1. (b) Lowest Unoccupied Molecular Orbital (LUMO) calculated for 1 using DFT calculations. (c) X-ray crystal structure of an asymmetric bis(m-oxo)dicopper complex prepared by reaction of 1 with [(TMPDA)Cu(I)(CH3CN)]O3SCF3 (TMPDA = N,N,N,N-tetramethylethylenediamine). Key for X-ray structures: green = Cu, blue = N, red = O, gray = C. | |
|
|