

 |
 |
Comp Chem Research Developments | |
| Archive of Comp Chem Research News | |
July 10, 2002
|
|
|
|
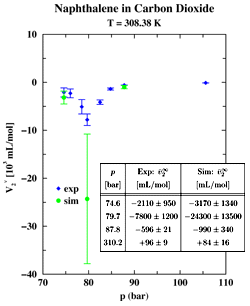
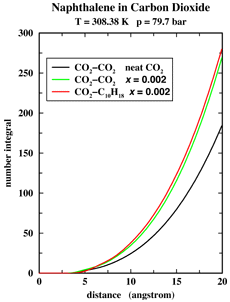
The critical point marks the high-temperature end of the vapor-liquid coexistence line in a pressure-temperature phase diagram and the supercritical fluid (SCF) region is defined as the part of the phase diagram characterized by temperatures and pressures higher than the critical temperature and pressure, respectively. More than 100 years ago, Hanny and Hogarth discovered the ability of SCFs to act as powerful solvents. However, it took until the late 1970s for SCF extraction to become an industrially viable process. The most famous example of a SCF extraction is the decaffeination of green coffee beans with supercritical carbon dioxide as the solvent. Currently, SCFs are also used in the removal of nicotine from tobacco, the extraction of hops, flavors, spices, and perfumes from natural products, the extraction of lower boiling products from the residues of crude oil distillation, and for separating solvents and monomers from polymers. Despite the great promise for SCF technology, a fundamental understanding of SCFs is still lacking. Of great interest is whether the distribution of supercritical solvents is relatively homogeneous on the microscopic scale, or whether there is a pronounced clustering of solvent molecules around solutes. In particular, pioneering measurements by Eckert and co-workers [J. Phys. Chem. 90, 2738 (1986)] have demonstrated large NEGATIVE partial molar volumes for solutes at infinite dilution in supercritical carbon dioxide. These measurements are usually interpreted in terms of a local solvent density enhancement. Undergraduate student Dylan Drake-Wilhelm and Professor Ilja Siepmann of the Department of Chemistry have carried out Monte Carlo simulations to investigate the microscopic origin of the large negative partial molar volumes found in dilute solutions of naphthalene in supercritical carbon dioxide (see Figure 1). From calculations of the number of solvent molecules (called number integral) surrounding a given solute or solvent molecule (see Figure 2) we can see that the addition of a small amount of naphthalene leads to an overall increase in the solvent density by about 30%, but that the solvation environments seen by carbon dioxide or naphthalene are very similar. Thus the large partial molar volumes are caused by a uniform long-range condensation and NOT by local density enhancements. |
|
 |
 |
|
The development of advanced computational strategies for the most challenging problems in chemistry and chemical physics is a theme common to the research endeavors of the Minnesota Computational Chemistry Group, where research includes new theoretical formulations, the development of new computational algorithms, and use of state-of-the-art supercomputers to solve prototype problems to high accuracy and to predict chemically useful results for a wide range of system scales ranging from a few atoms to thousands of atoms. Financial support from the National Science Foundation, Divisions of Chemical and Transport Systems and of Analytical and Surface Chemistry, is gratefully acknowledged. Part of the computer resources were provided by the Minnesota Supercomputing Institute. |
|
|
| |