

 |
 |
Comp Chem Research Developments | |
| Archive of Comp Chem Research News | |
November 14, 2001
|
|
|
|
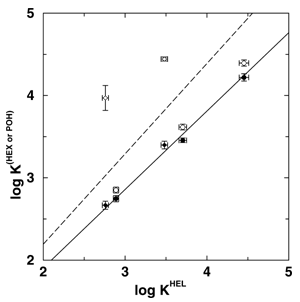
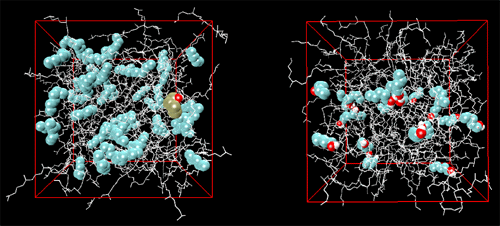
Gas-liquid chromatography (GC) is of vast importance for the separation, identification, and quantification of volatile chemicals from a host of sources which include environmental, industrial, biological, forensic, and other samples. The basis of GC has long been understood in terms of vapor-liquid phase equilibria for a solute between a gaseous mobile phase and a high boiling liquid-like stationary phase with strong solute-stationary phase interactions. Concentration effects, which lead to a degradation of the peak shape and to a loss of efficiency in GC, are usually explained in macroscopic terms of an ``isotherm effect'', i.e., the equilibrium distribution of a solute between gas and stationary phase is expressed as a function of solute concentration and temperature. Molecular simulations were performed by graduate student Collin Wick, Professor Ilja Siepmann and Dr. Mark Schure (of Rohm & Haas) to elucidate the molecular basis for concentration (isotherm) effects on retention in GC and, in particular, the changes in analyte partitioning caused by overloading a model chromatographic system with either an alkane or an alcohol. Squalane was used as the stationary phase material, and the analytes included n-pentane, n-hexane, n-heptane, 1-butanol, and 1-pentanol. Three systems were studied that differed in the mobile phase composition: (i) a helium vapor (system HEL), (ii) a n-hexane vapor (system HEX), and (iii) a 1-pentanol saturated helium vapor (system POH). While the amount of helium that partitions into the stationary phase is very small, both n-hexane and 1-pentanol partition strongly into and thereby swell the stationary phase. Although the swelling of the stationary phase leads to a reduction in the partition coefficients for the alkane solutes for both the n-hexane and 1-pentanol swollen stationary phases, the effects on the alcohol solutes differ markedly (see Figure 1). Whereas saturation by $n$-hexane causes a decrease of the alcohol partition contants (to a similar extent as for the alkane solutes), the saturation by 1-pentanol causes a dramatic increase of the alcohol partition coefficients. The formation of hydrogen-bonded alcohol aggregates in the liquid phase is the microscopic origin for the dramatic effect of 1-pentanol saturation on the retention of alcohols (see Figure 2). A detailed description of this research will appear in Analytical Chemistry (Dec. 2001). |
|
 |
|
Scatter plot of the logarithm of the partition coefficients for the alkane (circles) and alcohol (diamonds) solutes in systems HEX (filled symbols) and POH (open symbols) versus the logarithm of the partition coefficients for system HEL. The lines represent linear fits (constrained to pass through the origin) and the slopes and correlation coefficients are s=0.95 and r=0.99 (system HEX, solid line) and s=1.10 and r=0.55 (system POH, dashed line). | |
 |
|
Snapshots of the squalane phases for systems HEX (left, boxlength of 4.62 nm) and POH (right, boxlength of 4.48 nm). The overloaded analyte molecules are depicted by cyan, red, and white spheres for methyl/methylene groups, oxygen, and hydrogen atoms, respectively, whereas a stick representation is used for the squalane molecules. The snapshot for system HEX contains also a 1-pentanol solute highlighted by brown spheres for the methyl/methylene groups. | |
The development of advanced computational strategies for the most challenging problems in chemistry and chemical physics is a theme common to the research endeavors of the Minnesota Computational Chemistry Group, where research includes new theoretical formulations, the development of new computational algorithms, and use of state-of-the-art supercomputers to solve prototype problems to high accuracy and to predict chemically useful results for a wide range of system scales ranging from a few atoms to thousands of atoms. Financial support from the National Science Foundation, Divisions of Chemical and Transport Systems and of Analytical and Surface Chemistry, is gratefully acknowledged. Part of the computer resources were provided by the Minnesota Supercomputing Institute. |
|
|
|