

 |
 |
Comp Chem Research Developments | |
| Archive of Comp Chem Research News | |
September 6, 2000
|
|
Knowledge of the 1-octanol/water partition coefficient, KOW, and the corresponding Gibbs free energy of transfer, DG = - R T ln KOW, for a given solute is the main ingredient of quantitative structure-activity relationships and has been used to correlate or predict a plethora of solute properties, including the pharmacokinetic characteristics of drug compounds in biophases (membranes, adipose tissue, and body fluids) and the toxicity and transport of pollutants in soil/groundwater systems. The reasoning behind this is that the partitioning of a solute molecule (e.g., drug or pollutant) between a polar aqueous environment and a non-polar organic environment (e.g., biological membrane or soil) is most often the rate-determining step in biological, environmental, and geological processes. 1-octanol consists of a hydrophilic head and a lipophilic tail leading to a microheterogeneous solvent phase which has been found to mimic particularly well the complexities of biological and other environments. It has been estimated that experimental KOW are now available for more than 18000 solutes, but this is nowhere close to the number of possible drug molecules that can be synthesized by combinatorial chemistry approaches or possible pollutants. In addition, little is known about the effect of water saturation on the solvent characteristics of wet 1-octanol. 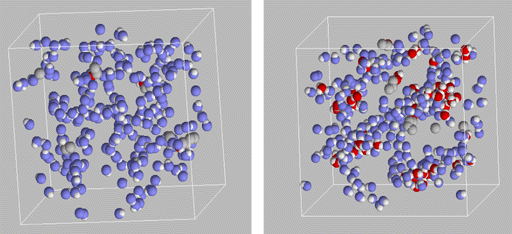
Figure 1: Snapshots of dry and water-saturated 1-octanol phases. The gray, blue, red, and white spheres depict alkyl groups of solute molecules, oxygen atoms of 1-octanol, oxygen atoms of water or solute molecules, and hydrogen atoms, respectively. The alkyl tails of 1-octanol are omitted for clarity. Using computer resources provided by the Minnesota Supercomputing Institute, graduate student Bin Chen and Professor Ilja Siepmann of the Department of Chemistry have carried out Monte Carlo simulations to investigate the partitioning of normal alkane and primary alcohol solutes between water and (dry or wet) 1-octanol phases. These simulations make use of the MC3S (Monte Carlo for Complex Chemical Systems) molecular modeling package and of the TraPPE (Transferable Potentials for Phase Equilibria) force field which are both under development in the Siepmann research group. In particular, configurational-bias Monte Carlo simulations in the Gibbs ensemble allow for the precise determination of DG from the ratio of the solute number densities in the two coexisting phases. Comparison of the partitioning between a helium vapor phase and dry and wet 1-octanol established that water saturation affects mostly the partitioning of polar solutes, while differences for alkane partitioning were found to be negligible. Agreement with experimental data by the group of Professor Peter Carr is very good. Analysis of the microscopic-level structure of the wet 1-octanol phase (see Figure 1) shows preferential partitioning of short alcohols (methanol and ethanol) into the water-rich regions of the microheterogeneous solvent mixture, whereas 1-butanol solutes are found preferentially at the boundary of hydrogen-bonded clusters. A brief description of this research work has been published in The Journal of the American Chemical Society2000, 122, 6464-6467. Financial support from the National Science Foundation is gratefully acknowledged. Bin Chen would like to thank the Graduate School, University of Minnesota, for the award of a Stanwood Johnston Memorial Fellowship and a Doctoral Dissertation Fellowship. |
|
|
| |