

 |
 |
Comp Chem Research Developments | |
| Archive of Comp Chem Research News | |
February 9, 2000
Single-electron transfer steps are often involved as the rate-determining step in reaction pathways that lead to the transformation of certain classes of anthropogenic organic compounds in the environment. A key molecular descriptor in modeling electron-transfer kinetics is the one-electron redox potential. Graduate student Paul Winget and Professors Chris Cramer and Don Truhlar, together with Dr. Eric Weber of the Environmental Protection Agency, have demonstrated the utility of pure computational techniques (involving ab initio or semiempirical electronic structure theory and quantum mechanical continuum solvation models) and of certain kinds of linear free energy relationships for predicting the 1-electron oxidation potentials of substituted anilines (Physical Chemistry Chemical Physics 1999, in press). Mean accuracies from 20 to 90 mV over 21 different substituted anilines were achieved with different approaches. The figure below illustrates use of a free energy cycle to compute such an oxidation potential in aqueous solution. With Professor Eric Patterson of Truman State University, Professors Cramer and Truhlar have applied this same technology to characterize the reaction path by which hexachloroethane (a common contaminant of drinking water) is transformed in the environment to tetrachloroethylene (Environmental Science & Technology, submitted for publication). 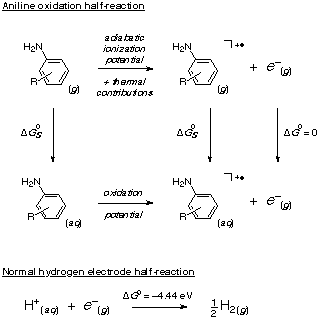 |
|
|
| |