

 |
 |
Comp Chem Research Developments | |
| Archive of Comp Chem Research News | |
February 23, 2000
The ability of supercritical fluids (SCFs) to act as powerful solvents was discovered more than 100 years ago, but it took until the late 1970s for SCF extraction to become an industrially viable process. The most famous example of a SCF extraction is the decaffeination of green coffee beans with supercritical CO2 as the solvent. Currently, SCFs are also used in the removal of nicotine from tobacco, the extraction of hops, flavors, spices, and perfumes from natural products, the extraction of lower boiling products from the residues of crude oil distillation, and for separating solvents and monomers from polymers. Despite the great promise for SCF technology, a fundamental understanding of SCFs is still lacking. Of great interest is whether the distribution of supercritical solvents is relatively homogeneous on the microscopic scale, or whether there is a pronounced clustering of solvent molecules around solutes. Graduate students Marcus Martin and Bin Chen and Professor Ilja Siepmann used configurational-bias Monte Carlo simulations in the Gibbs ensemble to calculate pressure-composition (see Figure) and temperature-composition diagrams for the binary mixture of n-heptane and supercritical ethane (a prototypical example for a supercritical fluid extraction system). The applicability of an SCF extraction can be determined from knowledge of these phase diagrams which provide, among other things, information on the dependence of SCF solvent properties on temperature and pressure. It is very encouraging that the molecular simulations can quantitatively predict the SCF phase equilibria without using any special binary mixing parameters.
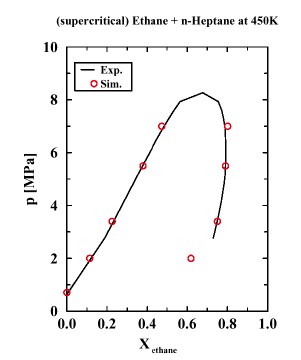 Analysis of the microscopic structure of the SCF phase does not support a supercritical solvation mechanism which involves preferential solvation of the solute (n-heptane) by the supercritical solvent (ethane) or which requires non-random mixing of the two components. It is argued here that changes in both the supercritical and liquid phases contribute significantly to the enhanced solubility of n-heptane in high-pressure supercritical ethane: Ethane partially replaces n-heptane in the liquid phase (``pushing'') and ethane acts as a non-specific solvent in the supercritical phase (``pulling'').
A full description of this research work will appear in The Journal of
Physical Chemistry (March 16, 2000 issue). Financial support from the
National Science Foundation is gratefully acknowledged. We would like
to thank the Department of Energy for a Computational Science Graduate
Fellowship (MGM), and the Graduate School, University of Minnesota,
for the award of a Stanwood Johnston Memorial Fellowship (BC). Part
of the computer resources were provided by the Minnesota
Supercomputing Institute.
|
|
|
| |