The prediction of phase equilibria of multicomponent
mixtures is one of the grand challenges for molecular simulation
requiring both accurate force
fields and efficient sampling
algorithms.
 Gas expanded liquids. Expansion of an
organic solvent by an inert gas can be used to tune the solvent's liquid
density, solubility strength, and transport properties. In particular,
gas expansion can be used to induce miscibility at low temperatures for
solvent combinations that are biphasic at standard pressure, providing a
new route to enhance reaction rates for biphasic catalytic systems.
Graduate student Ling
Zhang and Professor Ilja Siepmann have
used particle-based simulations to investigate the vapor-liquid-liquid
equilibria and the microscopic structures for the ternary mixture of
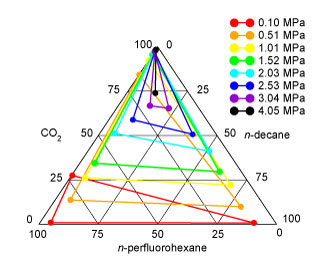
n-decane, n-perfluorohexane, and carbon dioxide. From
the predicted phase diagram shown above, one can see that the two liquid
phases are almost immiscible at atmospheric pressure (horizontal tie
line). At elevated pressures, carbon dioxide swells the two liquid
phases (the horizontal tie lines move upward to higher carbon dioxide
concentrations), and these expanded phases become more miscible. Above
the upper critical solution pressure (about 3.3 MPa in good agreement
with experiment), there is a single, albeit on a microscopic-level
heterogeneous liquid phase.
Gas expanded liquids. Expansion of an
organic solvent by an inert gas can be used to tune the solvent's liquid
density, solubility strength, and transport properties. In particular,
gas expansion can be used to induce miscibility at low temperatures for
solvent combinations that are biphasic at standard pressure, providing a
new route to enhance reaction rates for biphasic catalytic systems.
Graduate student Ling
Zhang and Professor Ilja Siepmann have
used particle-based simulations to investigate the vapor-liquid-liquid
equilibria and the microscopic structures for the ternary mixture of
n-decane, n-perfluorohexane, and carbon dioxide. From
the predicted phase diagram shown above, one can see that the two liquid
phases are almost immiscible at atmospheric pressure (horizontal tie
line). At elevated pressures, carbon dioxide swells the two liquid
phases (the horizontal tie lines move upward to higher carbon dioxide
concentrations), and these expanded phases become more miscible. Above
the upper critical solution pressure (about 3.3 MPa in good agreement
with experiment), there is a single, albeit on a microscopic-level
heterogeneous liquid phase.
 Polymeric surfactants in supercritical carbon
dioxide. Supercritical carbon dioxide has tremenduous potential
as a versatile, environmentally benign process solvent. The biggest
factor hindering its wide use is its low solvent power, requiring the
addition of entrainers or surfactants to enhance the solubility of polar
solutes. While partially fluorinated surfactants possess desirable
solubility characteristics, their cost is prohibitive and their
environmental impact is not fully understood. Therefore, the development
of cheaper and more benign hydrocarbon-based polymeric surfactants is
highly desirable and necessary for carbon dioxide to become an
economically viable solvent for a variety of processes. The search for
novel polymeric surfactants is hindered by synthetic challenges and
incomplete understanding of the molecular interactions and thermodynamic
parameters that control desirable solubility characteristics for
polymeric surfactants. Following upon pioneering experimental work by Eric Beckman and
co-workers on carbonate ether copolymers, postdoctoral fellow Collin
Wick in collaboration with Professors Doros Theodorou and
Ilja Siepmann
have demonstrated that molecular simulations can be employed to
accurately predict the phase equilibria of a CO
Polymeric surfactants in supercritical carbon
dioxide. Supercritical carbon dioxide has tremenduous potential
as a versatile, environmentally benign process solvent. The biggest
factor hindering its wide use is its low solvent power, requiring the
addition of entrainers or surfactants to enhance the solubility of polar
solutes. While partially fluorinated surfactants possess desirable
solubility characteristics, their cost is prohibitive and their
environmental impact is not fully understood. Therefore, the development
of cheaper and more benign hydrocarbon-based polymeric surfactants is
highly desirable and necessary for carbon dioxide to become an
economically viable solvent for a variety of processes. The search for
novel polymeric surfactants is hindered by synthetic challenges and
incomplete understanding of the molecular interactions and thermodynamic
parameters that control desirable solubility characteristics for
polymeric surfactants. Following upon pioneering experimental work by Eric Beckman and
co-workers on carbonate ether copolymers, postdoctoral fellow Collin
Wick in collaboration with Professors Doros Theodorou and
Ilja Siepmann
have demonstrated that molecular simulations can be employed to
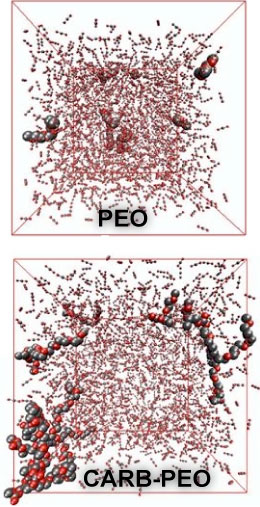
accurately predict the phase equilibria of a CO2-philic
hydrocarbon surfactant with CO2 and to show that the
accessibility of the carbonyl oxygens plays a major role for the higher
solubility of a carbonate poly(ethylene oxide) (CARB-PEO) copolymer
compared to a poly(ethylene oxide) (PEO) of similar molecular weight.
The findings of this work suggest that the accessible surface area of
polar groups (oxygen or fluorine) should be taken into account as a
design element for the development of CO2-philic
surfactants.



