

 |
 |
Comp Chem Research Developments | |
| Archive of Comp Chem Research News | |
|
July 21, 2004 | |
| |
|
Insight into the mechanism of RNA catalysis holds promise in the design
of new medical therapies that target genetic disorders as well as the
development of new biotechnology. One experimental method used to
probe the mechanism of RNA enzymes is the measurement of so-called thio effects: the
change in reaction rate that occurs upon substitution of key phosphoryl
oxygen positions with sulfur. The mechanstic interpretation of
experimental thio effects, however, is often ambiguous. Theoretical
methods offer a powerful tool to aid in the mechanistic interpretation of
experimental thio effects and provide additional insight into the chemical
reaction dynamics. Recently, graduate student Brent Gregersen and Professor Darrin York of the Department of Chemistry, in collaboration with MSI research scholar Prof. Xabier Lopez of the University of the Basque Country, reported results of a series of activated dynamics simulations of thio effects on the transesterification reaction of an RNA sugar-phosphate model in solution (Fig. 1). | |
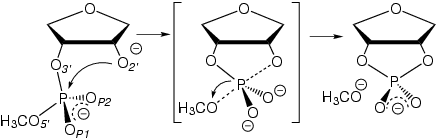 | |
| Figure 1:
In-line dianionic mechanism
of transphosphorylation. | |
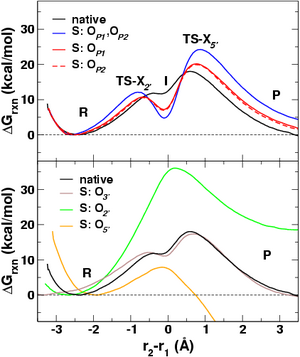
| The simulations employed a new combined quantum mechanical/molecular mechanical approach, with rigorous treatment of electrostatic interactions, to determine the reaction free energy profiles in solution (Fig. 2a). The change in solvent structure as a function of the reaction coordinate provides a detailed microscopic picture of the role of solvent in the reactions (Fig. 2b). These results are of fundamental biological importance and have been published in a recent issue of the Journal of the American Chemical Society [JACS, 126, 7504 (2004)]. Results of this work can be compared to those contained in QCRNA, a new on-line database of high-level quantum chemical data for RNA catalysis developed by the York Group. | |
| (a) |
(b) |
 |
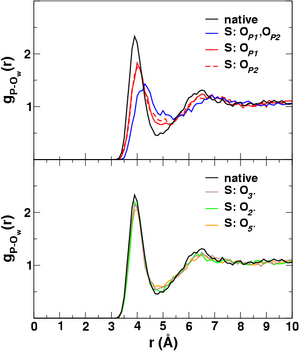 |
| Figure 2:
(a) Free energy
profiles for native and thio-substituted transphosphorylation reactions.
(b) Radial distribution of water
oxygens (OW) around phosphorus (P) at the transition
state. | |
|
| |