

 |
 |
Comp Chem Research Developments | |
| Archive of Comp Chem Research News | |
| December 24th, 2003 | |
| |
|
Human immunodeficiency virus type 1 (HIV-1) nucleocapsid protein (NC) plays several important roles in the viral life cycle and presents an attractive therapeutic target. Quantum chemical calculations offer great promise as tools for macromolecular characterization that are key in problems of rational drug design. These electronic structure methods provide valuable insight into the polarized electrostatic features and riogioselective chemical reactivity of molecules. Conventional quantum electronic structure methods, however, are not capable of handling very large, complex systems like solvated biological macromolecules because the computational effort scales non-linearly with the system size. The York Group has pioneered new linear-scaling electronic structure methods that circumvent the scaling bottleneck of conventional quantum methods and allow applications to be extended to very large systems (tens of thousands of atoms) in solution. Recently, graduate student Jana Khandogin and Professor Darrin York, in collaboration with Professor Karin Musier-Forsyth of the Chemistry Department, have characterized the macromolecular reactivity of NC and its binding to RNA through determination of electrostatic and chemical descriptors derived from linear-scaling quantum calculations in solution (Fig. 1). The computational results offer a rationale for the experimentally observed susceptibility of the Cys49 thiolate toward small-molecule electrophilic agents, and support the recently proposed stepwise protonation mechanism of the C-terminal Zn-coordination complex. The distinctive binding mode of NC to SL2 and SL3 stem-loops of the HIV-1 genomic RNA packaging signal is studied on the basis of protein side-chain contributions to the electrostatic binding energies. These results indicate the importance of several basic residues in the 3-10 helical region and the N-terminal zinc finger, and rationalize the presence of several evolutionarily conserved residues in NC. The combined reactivity and RNA binding study provides new insights that may contribute toward the structure-based design of anti-HIV therapies. These results have been published in the Journal of Molecular Biology 330, 993-1004 (2003). The work represents a key step in the ongoing research of the York Group focused on the design and application of theoretical methods to study RNA. | |
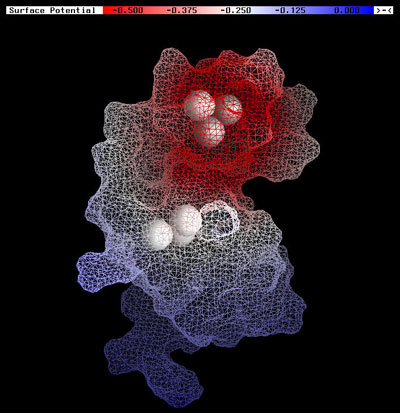 Fig. 1: Local hardness map of unbound HIV-1 NC protein (in SL3 -bound conformation). The red color clearly distinguishes the reactivity of the two zinc knuckles (shown as groups of white spheres) in accord with experimental observations that the N-terminal site (top) is more reactive toward electrophilic agents. | |
|
| |