

 |
 |
Comp Chem Research Developments | |
| Archive of Comp Chem Research News | |
| December 10th, 2003 | |
|
| |
|
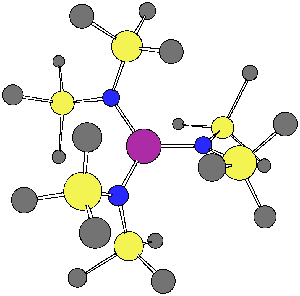
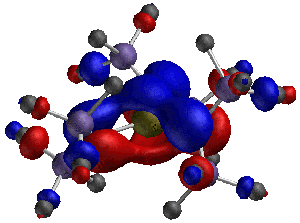
Group-13/Group-15 solids are well known as semiconductor materials. The synthesis of these materials can be accomplished from precursors where the Group-13 and Group-15 atoms are decorated with organic functional groups, and an understanding of the bonding and structures of these materials is a first step to predicting what solid structures may be constructed from them. In one recently published article in Inorganic Chemistry, 2003, 42, 6691, graduate student Bethany Kormos and Professor Christopher J. Cramer employed density functional theory to characterize the structures of various systems having the generic formula HmX(NR2)n where X was B, Al, Ga, or In; an example is shown below for m = 0, n =3, X = Ga, and R = SiMe3. The relative magnitudes of hyperconjugation and pi bonding over specific bonds in these systems were found to be surprisingly insensitive to m and n, but decreased quickly with the size of the Group 13 atom. In a related study published in Inorganic Chemistry, 2003, 42, 3431, graduate student Bing Luo and Professors Christopher J. Cramer and Wayne L. Gladfelter employed similar methods to explain the unusual ultraviolet spectrum of In[N(SiMe3)NMe2]3. The interaction of hydrazide lone pairs with a central pi system reminiscent of the classic non-Kekulé system trimethylenemethane (TMM) leads to an unusually narrow frontier orbital gap and a long wavelength absorption not seen in analogous amides. A particularly striking example of one of these twisted TMM-like orbitals is illustrated below. | |
  | |
|
| |