

 |
 |
Comp Chem Research Developments | |
| Archive of Comp Chem Research News | |
January 23, 2002
|
|
|
|
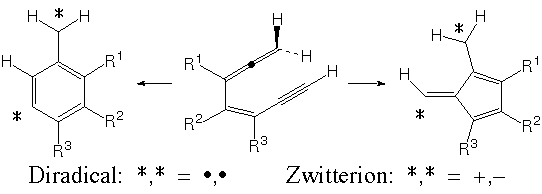
Christopher J. Cramer, Bethany L. Kormos, Mark Seierstad, Edward C. Sherer, and Paul Winget, Department of Chemistry and Supercomputing Institute, University of Minnesota. Certain antitumor drugs capable of cleaving double-helical DNA are generated in their active forms by cyclization of an internal enyne-allene to a diradical. However, both the structure of the final cyclization product and its DNA cleaving efficiency are strongly dependent on the nature of functional groups substituting the enyne-allene.
|
|
|
|
|
Density functional calculations carried out for several substituted enyne-allenes indicate that zwitterionic cyclization products, which are ineffective as DNA-cleaving agents, are strongly stabilized by substituents that can interact so as to neutralize one of the zwitterionic charges (e.g., an oxyanion as substituent R1 permits the product to cancel one positive and one negative charge by formation of an enone). In six-membered ring cyclizations, however, this effect is mitigated by aromaticity, which must be sacrificed to annihilate charge. These results should prove useful in design efforts aimed at improving efficiency in related pharmaceuticals. A preliminary communication of this work can be found in Organic Letters 2001, 3, 1881 |
|
|
| |