

 |
 |
Comp Chem Research Developments | |
| Archive of Comp Chem Research News | |
May 16, 2001
|
|
|
|
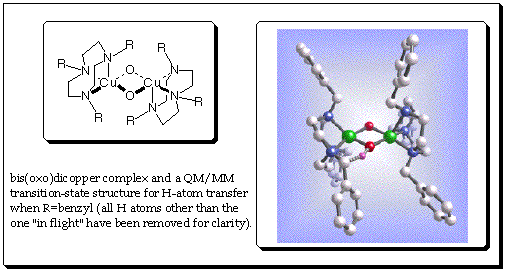
Youngshang Pak, Christopher Kinsinger, and Christopher J. Cramer, Department of Chemistry and Supercomputer Institute, University of Minnesota. Tolman and co-workers have demonstrated that bis(oxo)dicopper cores like the one shown below can exist in equilibrium with isomers where the two oxygen atoms are bonded (as a peroxide) and the copper atoms coordinate from either side in an h2 fashion--the latter isomers are known in certain metalloenzymes. They have also demonstrated that when R is a benzyl group, the complex self-destructs by intramolecular activation of a benzyl C-H bond in a reaction that is characterized by significant quantum mechanical tunneling of H affecting the rate constant. |
|
 |
|
Less clear from experiment is whether this rate-limiting reaction involves H-atom transfer, proton transfer, hydride transfer, decoupled individual electron and proton transfer steps, direct O-atom insertion into the C-H bond, or even possibly isomerization to the peroxo isomer prior to C-H bond activation. Using a combined quantum mechanics/molecular mechanics model, a transition state has been found that is consistent with the experimentally measured enthalpy of activation. Accounting for tunneling using a one-dimensional Eckart potential, predicted primary kinetic isotope effects are also in reasonable agreement with experiment. These results and additional analysis suggest that the reaction is well described as an H-atom transfer from carbon to oxygen in the bis(oxo)dicopper tautomer. A preliminary communication of this work can be found in Theoretical Chemistry Accounts 2001, 105, 477. Further characterization of the reaction coordinate is now under way. |
|
|
|