

 |
 |
Comp Chem Research Developments | |
| Archive of Comp Chem Research News | |
June 13, 2001
|
|
|
|
Mixtures of alkanes and alcohols are of great practical importance for the chemical and pharmaceutical industries. However, hydrogen-bonding of alcohols can lead to self-aggregation in the nonpolar alkane solvents, thereby causing strong deviations from ideal solution behavior. Graduate student John Stubbs and Professor Ilja Siepmann of the Department of Chemistry have performed configurational-bias Monte Carlo simulations in the isobaric-isothermal ensemble using the TraPPE--UA (transferable potentials for phase equilibria--united atom) force field to investigate the aggregation of dilute solutions of 1-hexanol in n-hexane. The spatial distribution of alcohols is sampled efficiently using special Monte Carlo moves. Snapshots of hexanol/hexane mixtures over a range of compositions and over a range of temperatures are shown in Figure 1. Analysis of the microscopic structures demonstrates super-strong aggregation (e.g., the radial distribution function for the 1% solution shows that the concentration of neighboring hydroxyl oxygens is enhanced by almost three orders of magnitude). There is a preference for the formation of tetramers and pentamers, and most of these aggregates possess a cyclic architecture. Overall agreement with experimental results is very satisfactory and the simulations allow to test various theorectical models used to interpret experimental results. |
|
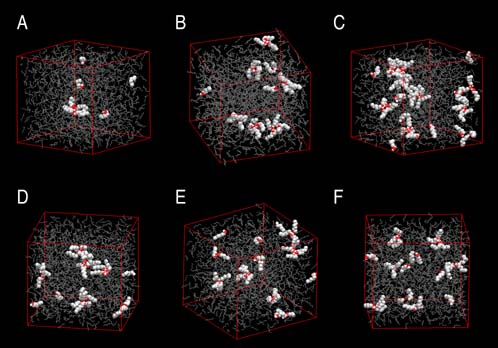 Snapshots of solutions of 1-hexanol in n-hexane (shown as spheres and sticks, respectively). Top panel: temperature of 298 K and mole fractions of 1%, 3%, and 5%. Bottom panel: Mole fraction of 3% and temperatures of 308 K, 318 K, and 328 K. |
|
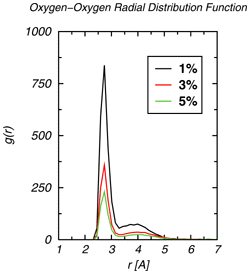 Oxygen--oxygen radial distribution functions for 1%, 3%, and 5% solutions at a temperature of 298 K. (A radial distribution function gives the probability to find a pair of atoms at a given separation normalized by the probability for a uniform system.) |
|
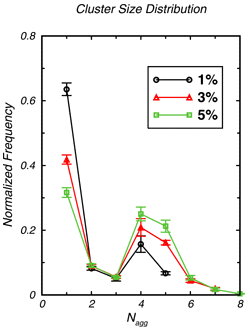 Distribution of hydrogen-bonded clusters over aggregation sizes for 1%, 3%, and 5% solutions at a temperature of 298 K. |
|
The development of advanced computational strategies for the most challenging problems in chemistry and chemical physics is a theme common to the research endeavors of the Minnesota Computational Chemistry Group, where research includes new theoretical formulations, the development of new computational algorithms, and use of state-of-the-art supercomputers to solve prototype problems to high accuracy and to predict chemically useful results for a wide range of system scales ranging from a few atoms to thousands of atoms. Financial support from the National Science Foundation, Divisions of Chemical and Transport Systems and of Analytical and Surface Chemistry, is gratefully acknowledged. Part of the computer resources were provided by the Minnesota Supercomputing Institute. |
|
|
|